Use RWE to Meet the Demands of Decision Makers
Use the Right Data to Make an Impact
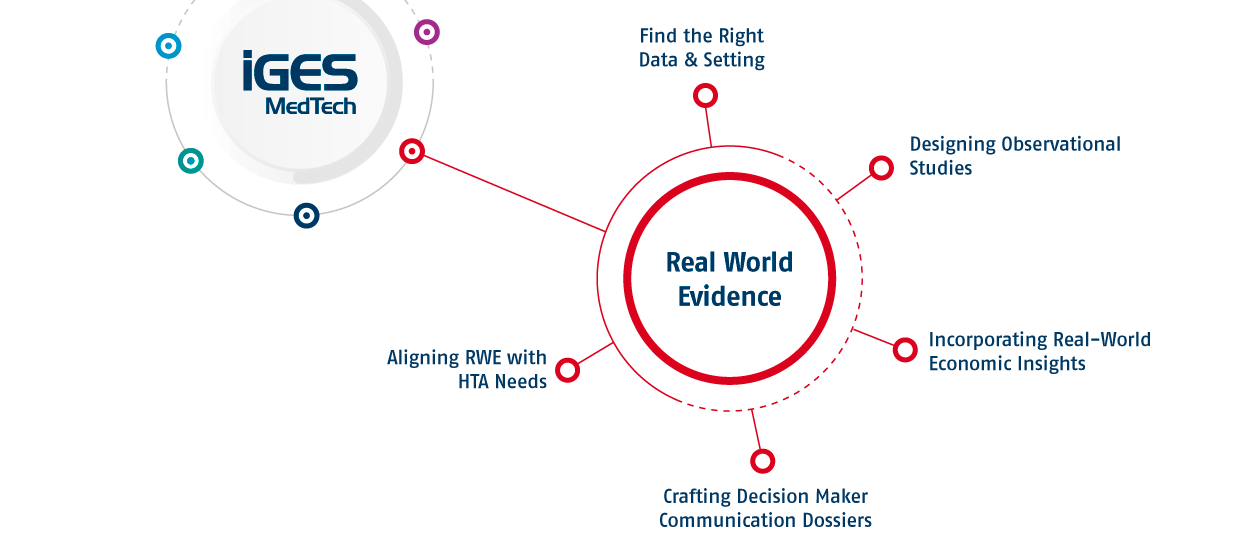
Real-World Evidence (RWE) is increasingly essential for demonstrating the clinical and economic value of medical devices. By integrating high-quality real-world evidence, companies can address the demands from decision makers. At IGES MedTech, we specialise in designing and executing RWE strategies, optimising the use of diverse data sets. From observational studies to leveraging economic models, we ensure your innovation aligns with decision maker expectations and regulatory requirements, paving the way for successful market access and long-term adoption.
To meet the expectations of decision makers regarding Real-World Evidence, it is crucial to work with data sources that have the right quality and scope. We help you identify the appropriate settings and data sources to achieve this.
Observational studies provide critical insights into how medical devices perform in real-world settings. IGES MedTech designs and implements robust observational studies that generate meaningful data on patient outcomes, safety, and effectiveness. These studies form a vital part of your evidence package, supporting decision maker negotiations and market positioning.
Economic models provide essential evidence to demonstrate cost-effectiveness and value to decision makers. At IGES MedTech, we integrate and interpret real-world economic models to highlight the financial and clinical impact of your innovation. Our expertise ensures these models are contextualised to meet the specific demands of European healthcare systems, strengthening your value proposition and decision maker negotiations.
Communicating the value of your device to decision makers requires clear, compelling evidence. IGES MedTech crafts targeted dossiers that highlight the clinical and economic benefits of your device. By aligning with decision maker priorities, we ensure your evidence resonates and facilitates approval processes.
Real-World Evidence may play a critical role in Health Technology Assessments when properly executed. IGES MedTech integrates RWE into your HTA submissions, ensuring your device meets the expectations of regulators and decision makers. Our approach bridges the gap between clinical trial data and real-world performance, strengthening your case for market access.
 Contact
Contact