Navigate Europe with Precision: Tailored Market Access Strategies
The First Step Towards Long-Term MedTech Success
A successful market launch begins with a well-defined strategy. Identifying the most promising markets, aligning with regulatory requirements, and addressing payer expectations are essential steps to saving time and resources while ensuring a strong foundation for success. At IGES MedTech, we specialise in crafting bespoke market access strategies tailored to your innovation’s unique value and the complexities of Europe’s healthcare systems. Our approach empowers you to navigate fragmented healthcare landscapes with confidence, accelerating your path to market and maximising patient impact.
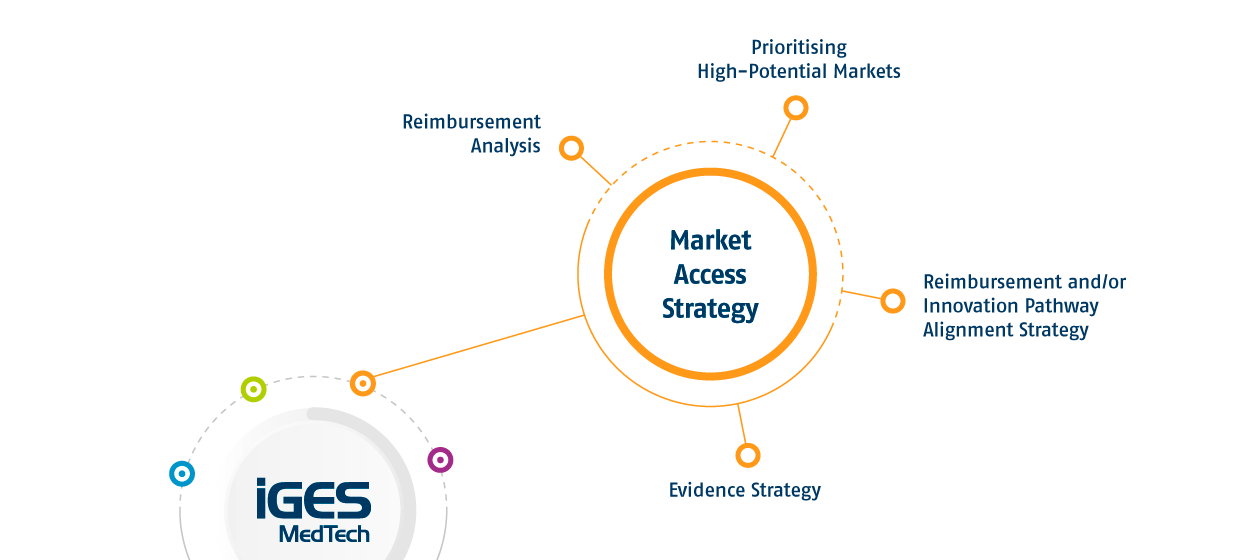
Understanding national reimbursement structures is essential for securing market access. IGES MedTech conducts in-depth analyses of reimbursement policies across Europe, identifying the most efficient routes to reimbursement and potential funding opportunities for your product.
Choosing the right markets is key to maximising your product’s impact and commercial success. IGES MedTech helps you evaluate opportunities across Europe’s healthcare systems, crafting entry strategies that prioritise the markets where your product can thrive.
Reimbursement is a critical factor in ensuring patient access and long-term market sustainability. In some cases, innovative medical devices can benefit from fast-track access through specific pathways. Our experts develop reimbursement and innovation pathway strategies that align with national healthcare frameworks and market demands, ensuring a seamless and efficient entry process.
A strong evidence strategy is fundamental to achieving reimbursement and market adoption. IGES MedTech supports the development of clinical and economic evidence plans, ensuring your product meets the expectations of decision-makers, payers, and healthcare providers across different markets.
 Contact
Contact